
Exhibit 99.2

NASDAQ:LTRN March 10 th , 2022 4:30 PM Eastern Time Fourth quarter 2021 Operating & Financial Results Conference Call / Webinar

NASDAQ:LTRN TODAY’S SPEAKERS Panna Sharma Chief Financial Officer and Secretary Chief Scientific Officer Investor Relations Chief Executive Officer, President and Director David Margrave Dr. Kishor Bhatia Nicole Leber 1

NASDAQ:LTRN This presentation contains forward - looking statements within the meaning of Section 27 A of the Securities Act of 1933 , as amended, and Section 21 E of the Securities Exchange Act of 1934 , as amended . These forward - looking statements include, among other things, statements relating to : future events or our future financial performance ; the potential advantages of our RADR® platform in identifying drug candidates and patient populations that are likely to respond to a drug candidate ; our strategic plans to advance the development of our drug candidates and antibody drug conjugate (ADC) development program ; estimates regarding the development timing for our drug candidates and ADC development program ; our research and development efforts of our internal drug discovery programs and the utilization of our RADR® platform to streamline the drug development process ; our intention to leverage artificial intelligence, machine learning and genomic data to streamline and transform the pace, risk and cost of oncology drug discovery and development and to identify patient populations that would likely respond to a drug candidate ; estimates regarding potential markets and potential market sizes ; sales estimates for our drug candidates and our plans to discover and develop drug candidates and to maximize their commercial potential by advancing such drug candidates ourselves or in collaboration with others . Any statements that are not statements of historical fact (including, without limitation, statements that use words such as "anticipate," "believe," "contemplate," "could," "estimate," "expect," "intend," "seek," "may," "might," "plan," "potential," "predict," "project," "target," “objective,” "aim,” “upcoming,” "should," "will," "would," or the negative of these words or other similar expressions) should be considered forward - looking statements . There are a number of important factors that could cause our actual results to differ materially from those indicated by the forward - looking statements, such as ( i ) the impact of the COVID - 19 pandemic, (ii) the risk that our research and the research of our collaborators may not be successful, (iii) the risk that we may not be able to successfully initiate, conduct, or conclude clinical testing for or obtain marketing approval for our product candidates ; (iv) the risk that no drug product based on our proprietary RADR A . I . platform has received FDA marketing approval or otherwise been incorporated into a commercial product, and (v) those other factors set forth in the Risk Factors section in our Annual Report on Form 10 - K for the year ended December 31 , 2021 , filed with the Securities and Exchange Commission on March 10 , 2022 . You may access our Annual Report on Form 10 - K for the year ended December 31 , 2021 under the investor SEC filings tab of our website at www . lanternpharma . com or on the SEC's website at www . sec . gov . Given these risks and uncertainties, we can give no assurances that our forward - looking statements will prove to be accurate, or that any other results or events projected or contemplated by our forward - looking statements will in fact occur, and we caution investors not to place undue reliance on these statements . All forward - looking statements in this presentation represent our judgment as of the date hereof, and, except as otherwise required by law, we disclaim any obligation to update any forward - looking statements to conform the statement to actual results or changes in our expectations . LTRN Forward Looking Statements 2

NASDAQ:LTRN Contents 01 Lantern Highlights 02 Financial Overview 03 R&D Updates 04 Q&A NASDAQ:LTRN 3
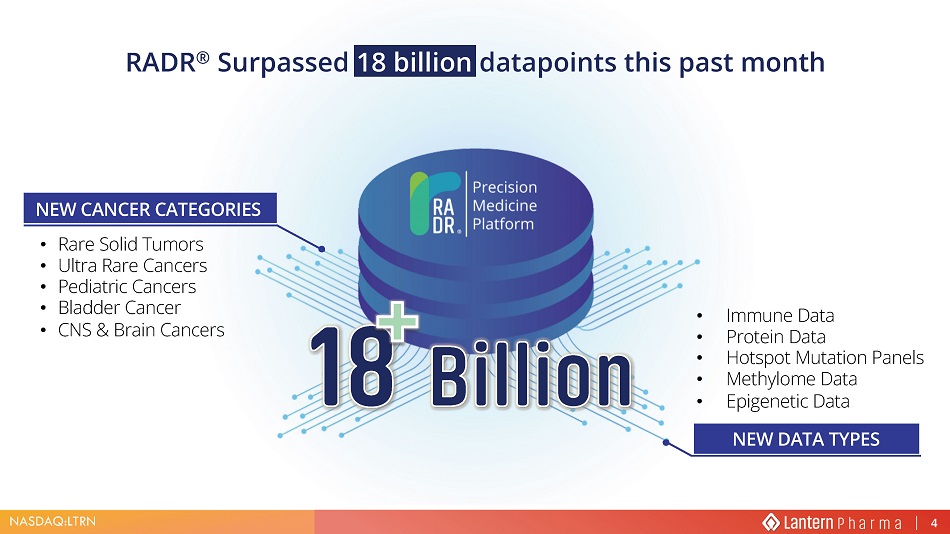
NASDAQ:LTRN NEW CANCER CATEGORIES RADR ® Surpassed 18 billion datapoints this past month • Rare Solid Tumors • Ultra Rare Cancers • Pediatric Cancers • Bladder Cancer • CNS & Brain Cancers • Immune Data • Protein Data • Hotspot Mutation Panels • Methylome Data • Epigenetic Data NEW CANCER CATEGORIES NEW DATA TYPES 4
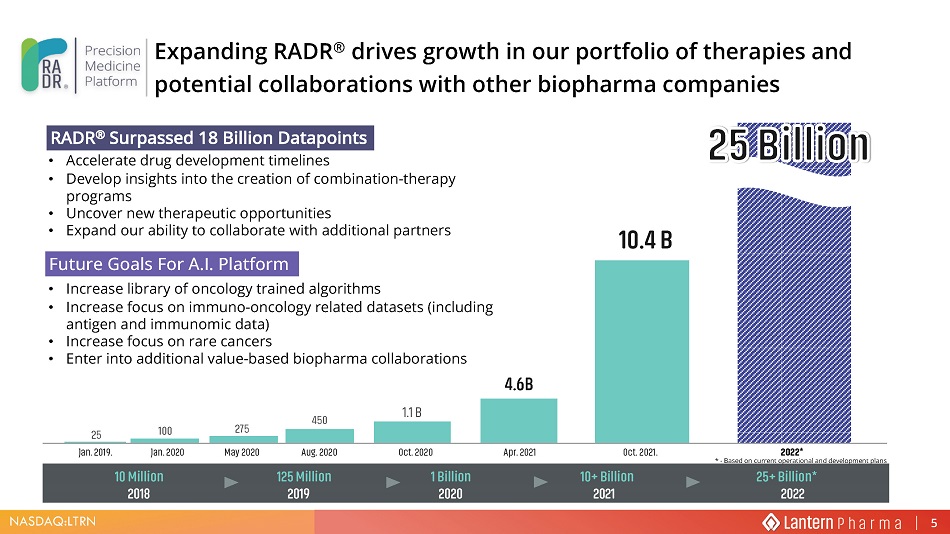
NASDAQ:LTRN * - Based on current operational and development plans 10 Million 125 Million 1 Billion 10+ Billion 25+ Billion* 2018 2019 2020 2021 2022 Jan. 2019. Jan. 2020 May 2020 Aug. 2020 Oct. 2020 Apr. 2021 Oct. 2021. 2022* 25 100 275 450 1.1 B 4.6B 10.4 B Future Goals For A.I. Platform RADR ® Surpassed 18 Billion Datapoints • Accelerate drug development timelines • Develop insights into the creation of combination - therapy programs • Uncover new therapeutic opportunities • Expand our ability to collaborate with additional partners • Increase library of oncology trained algorithms • Increase focus on immuno - oncology related datasets (including antigen and immunomic data) • Increase focus on rare cancers • Enter into additional value - based biopharma collaborations Expanding RADR ® drives growth in our portfolio of therapies and potential collaborations with other biopharma companies 5
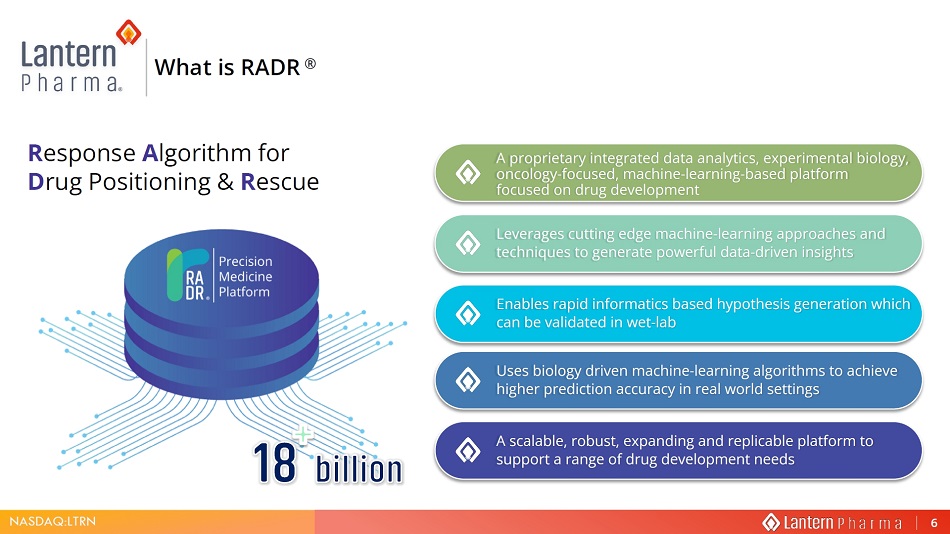
NASDAQ:LTRN R esponse A lgorithm for D rug Positioning & R escue What is RADR ® A proprietary integrated data analytics, experimental biology, oncology - focused, machine - learning - based platform focused on drug development Leverages cutting edge machine - learning approaches and techniques to generate powerful data - driven insights Enables rapid informatics based hypothesis generation which can be validated in wet - lab Uses biology driven machine - learning algorithms to achieve higher prediction accuracy in real world settings A scalable, robust, expanding and replicable platform to support a range of drug development needs 6
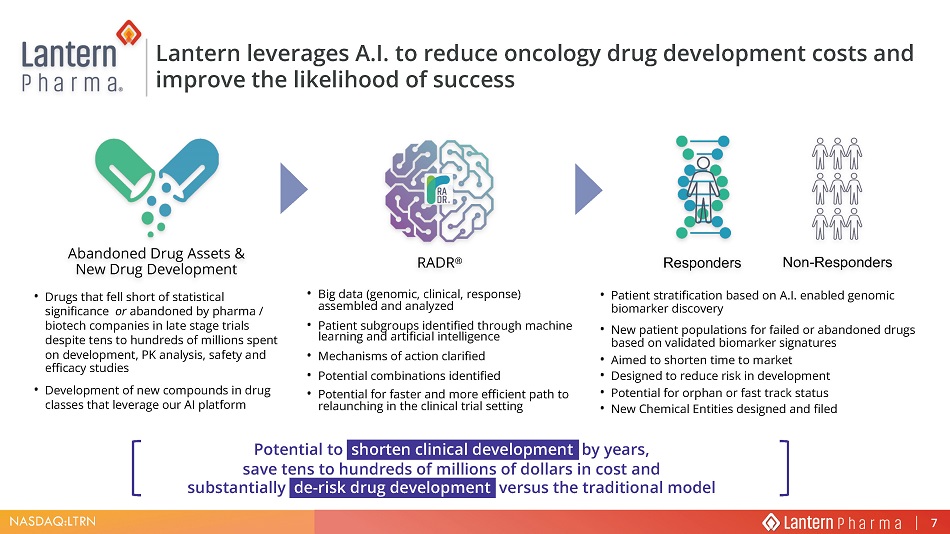
NASDAQ:LTRN • Drugs that fell short of statistical significance or abandoned by pharma / biotech companies in late stage trials despite tens to hundreds of millions spent on development , PK analysis, safety and efficacy studies • Development of new compounds in drug classes that leverage our AI platform • Big data (genomic, clinical, response) assembled and analyzed • Patient subgroups identified through machine learning and artificial intelligence • Mechanisms of action clarified • Potential combinations identified • Potential for faster and more efficient path to relaunching in the clinical trial setting • Patient stratification based on A.I. enabled genomic biomarker discovery • New patient populations for failed or abandoned drugs based on validated biomarker signatures • Aimed to shorten time to market • Designed to reduce risk in development • Potential for orphan or fast track status • New Chemical Entities designed and filed Abandoned Drug Assets & New Drug Development RADR ® Responders Non - Responders Lantern l everages A.I. to reduce oncology drug development cost s and improve the likelihood of success Potential to shorten clinical development by years, save tens to hundreds of millions of dollars in cost and substantially de - risk drug development versus the traditional model 7
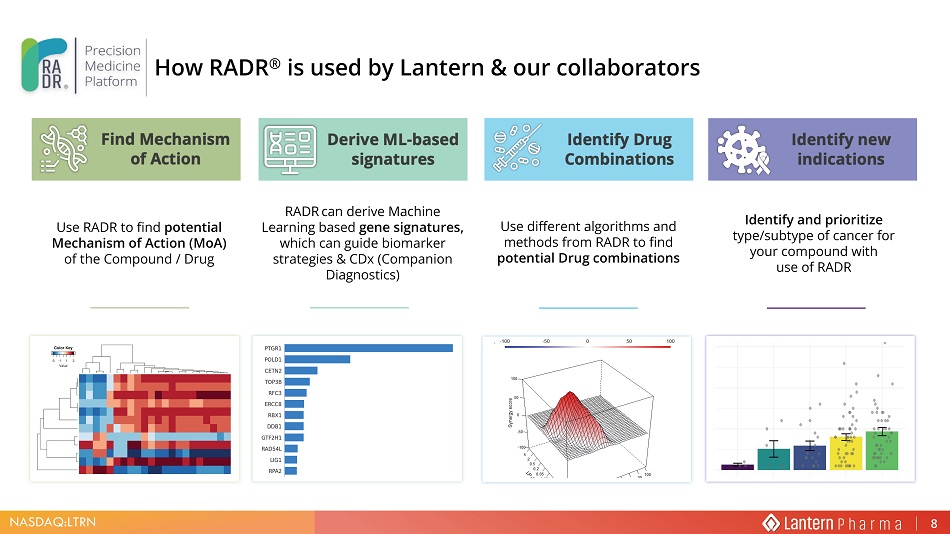
NASDAQ:LTRN Use RADR to find potential Mechanism of Action ( MoA ) of the Compound / Drug RADR can derive Machine Learning based gene signatures , which can guide biomarker strategies & CDx (Companion Diagnostics) Use different algorithms and methods from RADR to find potential Drug combinations Identify and prioritize type/subtype of cancer for your compound with use of RADR How RADR ® is used by Lantern & our collaborators 8
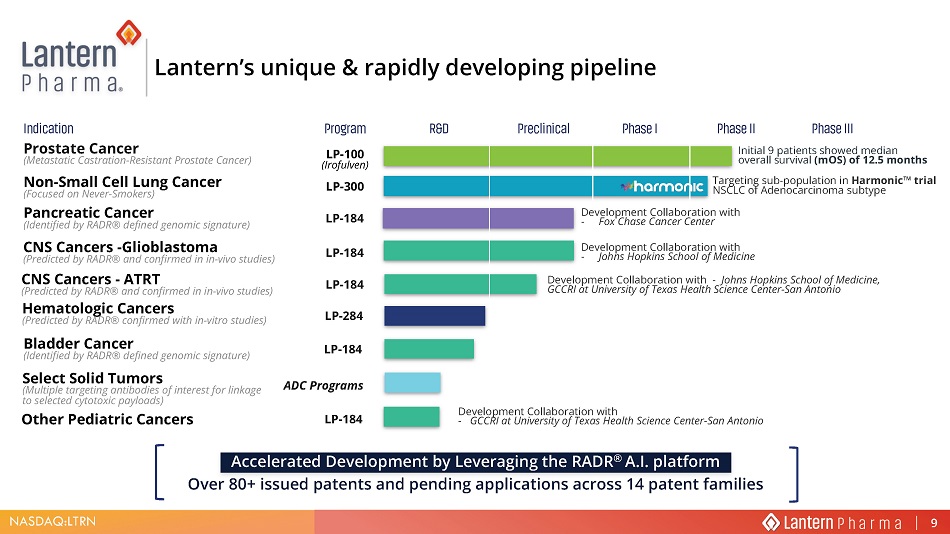
NASDAQ:LTRN Phase I Phase II Program Preclinical Indication Phase III R&D Lantern’s unique & rapidly d eveloping p ipeline ADC Programs Select Solid Tumors (Multiple targeting antibodies of interest for linkage to selected cytotoxic payloads) Phase I Phase II LP - 184 LP - 100 ( Irofulven ) LP - 300 Program Preclinical Indication Prostate Cancer (Metastatic Castration - Resistant Prostate Cancer) Non - Small Cell Lung Cancer (Focused on Never - Smokers) Bladder Cancer (Identified by RADR® defined genomic signature) Phase III R&D CNS Cancers - Glioblastoma (Predicted by RADR® and confirmed in in - vivo studies) LP - 184 LP - 284 Hematologic Cancers (Predicted by RADR® confirmed with in - vitro studies) Accelerated Development by Leveraging the RADR ® A.I. platform Over 80+ issued patents and pending applications across 14 patent families LP - 184 Pancreatic Cancer (Identified by RADR® defined genomic signature) CNS Cancers - ATRT (Predicted by RADR® and confirmed in in - vivo studies) LP - 184 7DUJHWLQJ VXE SRSXODWLRQ LQ +DUPRQLF Ƞ WULDO 16&/& RI $GHQRFDUFLQRPD VXEW\SH Development Collaboration with - Johns Hopkins School of Medicine Development Collaboration with - Johns Hopkins School of Medicine, GCCRI at University of Texas Health Science Center - San Antonio Initial 9 patients showed median overall survival ( mOS ) of 12.5 months Development Collaboration with - Fox Chase Cancer Center LP - 184 Other Pediatric Cancers Development Collaboration with - GCCRI at University of Texas Health Science Center - San Antonio
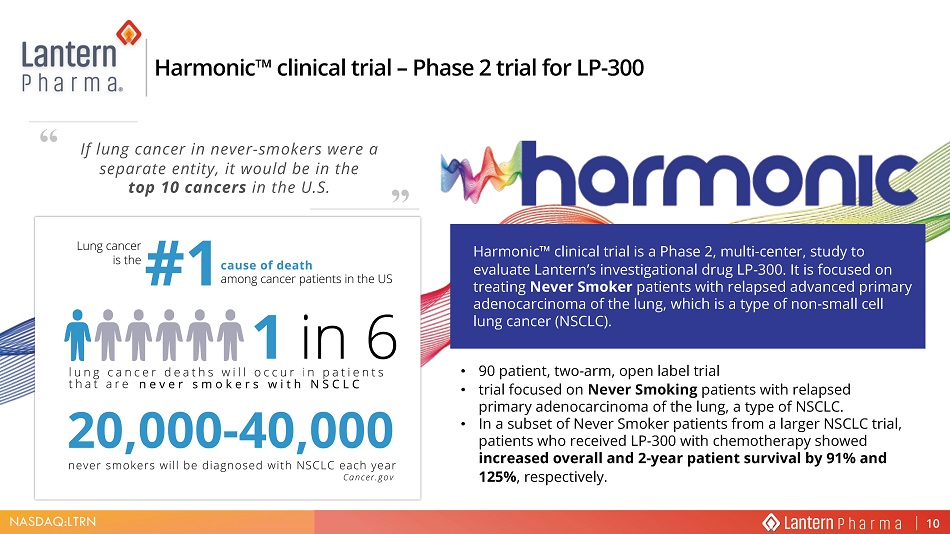
NASDAQ:LTRN • 90 patient, two - arm, open label trial • trial focused on Never Smoking patients with relapsed primary adenocarcinoma of the lung, a type of NSCLC. • In a subset of Never Smoker patients from a larger NSCLC trial, patients who received LP - 300 with chemotherapy showed increased overall and 2 - year patient survival by 91% and 125% , respectively. +DUPRQLF Œ FOLQLFDO WULDO ± 3KDVH WULDO IRU /3 +DUPRQLF Ƞ FOLQLFDO WULDO LV D 3KDVH PXOWL FHQWHU VWXG\ WR HYDOXDWH /DQWHUQ ȇ V LQYHVWLJDWLRQDO GUXJ /3 ΖW LV IRFXVHG RQ WUHDWLQJ 1HYHU 6PRNHU SDWLHQWV ZLWK UHODSVHG DGYDQFHG SULPDU\ DGHQRFDUFLQRPD RI WKH OXQJ ZKLFK LV D W\SH RI QRQ VPDOO FHOO OXQJ FDQFHU 16&/& If lung cancer in never - smokers were a separate entity, it would be in the top 10 cancers in the U.S. “ ”
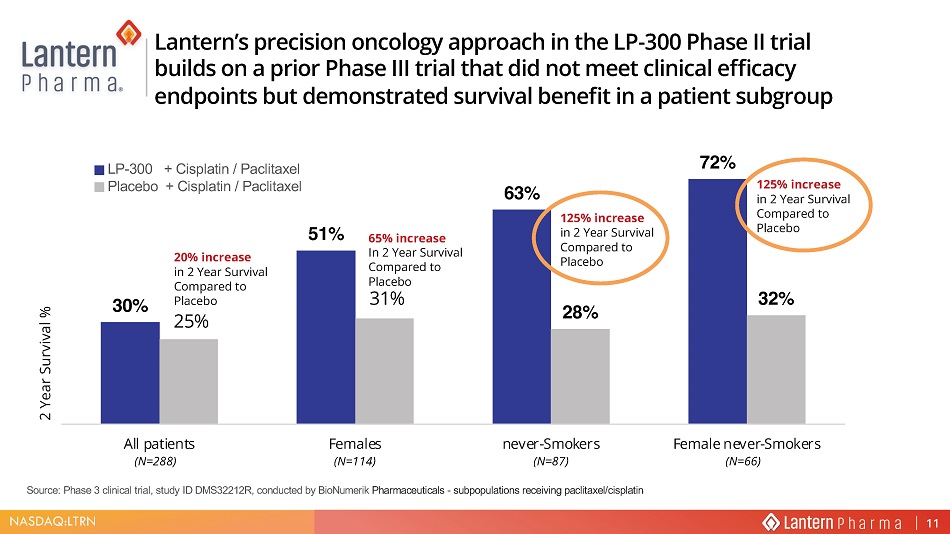
NASDAQ:LTRN Source: Phase 3 clinical trial, study ID DMS32212R, conducted by BioNumerik Pharmaceuticals - subpopulations receiving paclitaxel/cisplatin 30% 63% 72% 31% 32% All patients Females never-Smokers Female never-Smokers 2 Year Survival % LQFUHDVH LQ <HDU 6XUYLYDO &RPSDUHG WR 3ODFHER 125% increase in 2 Year Survival Compared to Placebo 65% increase In 2 Year Survival Compared to Placebo LQFUHDVH LQ <HDU 6XUYLYDO &RPSDUHG WR 3ODFHER (N=114) 1 (N=66) (N=288) /DQWHUQ ¶ V SUHFLVLRQ RQFRORJ\ DSSURDFK LQ WKH /3 3KDVH ,, WULDO EXLOGV RQ D SULRU 3KDVH ,,, WULDO WKDW GLG QRW PHHW FOLQLFDO HIILFDF\ HQGSRLQWV EXW GHPRQVWUDWHG VXUYLYDO EHQHILW LQ D SDWLHQW VXEJURXS LP - 300 + Cisplatin / Paclitaxel Placebo + Cisplatin / Paclitaxel 11
1$6'$4 /751 • Granted Orphan Drug Designation for the treatment of Pancreatic Cancer, GBM and ATRT • Granted Rare Pediatric Disease Designation for the treatment of ATRT • Positive preclinical data for LP - 184 in pancreatic cancer and GBM • Currently conducting IND enabling studies to support IND submission in 2022
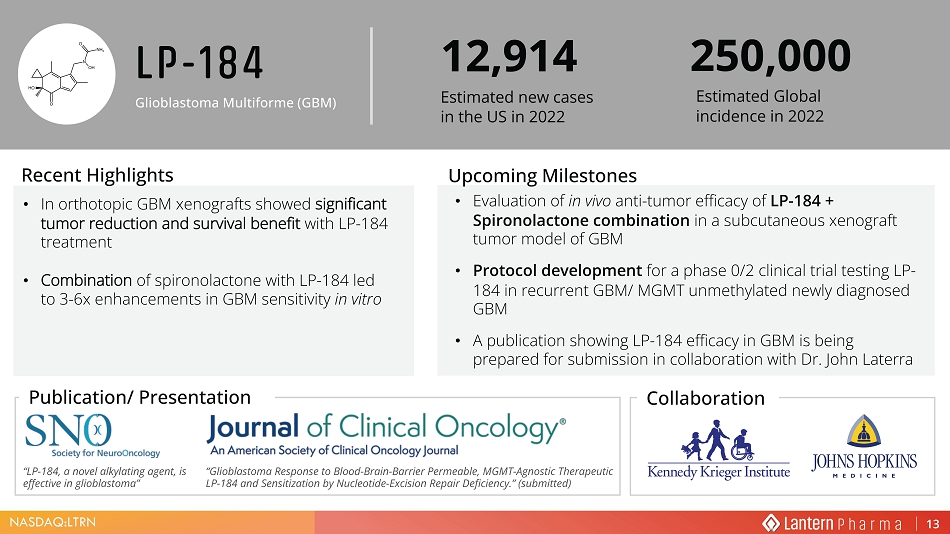
1$6'$4 /751 LP - 184 Glioblastoma Multiforme (GBM) ‡ ,Q RUWKRWRSLF *%0 [HQRJUDIWV VKRZHG VLJQLILFDQW WXPRU UHGXFWLRQ DQG VXUYLYDO EHQHILW ZLWK /3 WUHDWPHQW ‡ &RPELQDWLRQ RI VSLURQRODFWRQH ZLWK /3 OHG WR [ HQKDQFHPHQWV LQ *%0 VHQVLWLYLW\ LQ YLWUR • Evaluation of in vivo anti - tumor efficacy of LP - 184 + Spironolactone combination in a subcutaneous xenograft tumor model of GBM • Protocol development for a phase 0/2 clinical trial testing LP - 184 in recurrent GBM/ MGMT unmethylated newly diagnosed GBM • A publication showing LP - 184 efficacy in GBM is being prepared for submission in collaboration with Dr. John Laterra Recent Highlights 8SFRPLQJ 0LOHVWRQHV 3XEOLFDWLRQ 3UHVHQWDWLRQ 12,914 Estimated new cases in the US in 2022 (VWLPDWHG *OREDO LQFLGHQFH LQ 250,000 Collaboration “LP - 184, a novel alkylating agent, is effective in glioblastoma” Ȋ *OLREODVWRPD 5HVSRQVH WR %ORRG %UDLQ %DUULHU 3HUPHDEOH 0*07 $JQRVWLF 7KHUDSHXWLF /3 DQG 6HQVLWL]DWLRQ E\ 1XFOHRWLGH ([FLVLRQ 5HSDLU 'HILFLHQF\ ȋ VXEPLWWHG
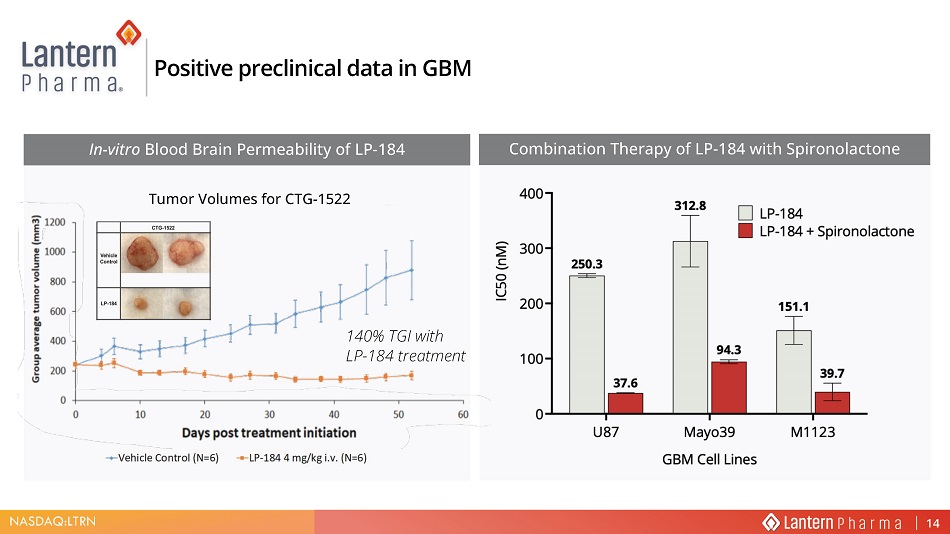
NASDAQ:LTRN 140% TGI with LP - 184 treatment 7XPRU 9ROXPHV IRU &7* In - vitro Blood Brain Permeability of LP - 184 Positive preclinical data in GBM Combination Therapy of LP - 184 with Spironolactone
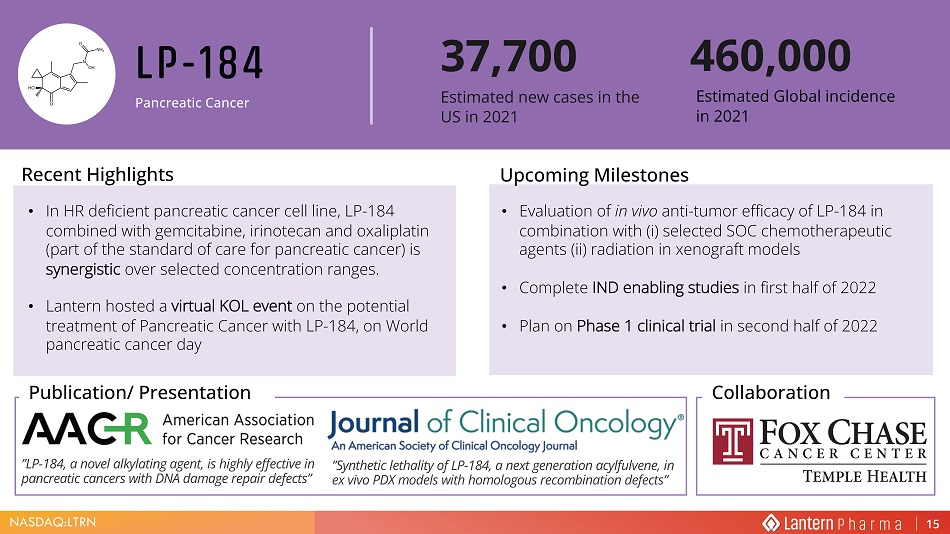
NASDAQ:LTRN >W Ͳ ϭϴϰ Pancreatic Cancer ‡ ,Q +5 GHILFLHQW SDQFUHDWLF FDQFHU FHOO OLQH /3 FRPELQHG ZLWK JHPFLWDELQH LULQRWHFDQ DQG R[DOLSODWLQ SDUW RI WKH VWDQGDUG RI FDUH IRU SDQFUHDWLF FDQFHU LV V\QHUJLVWLF RYHU VHOHFWHG FRQFHQWUDWLRQ UDQJHV ‡ /DQWHUQ KRVWHG D YLUWXDO .2/ HYHQW RQ WKH SRWHQWLDO WUHDWPHQW RI 3DQFUHDWLF &DQFHU ZLWK /3 RQ :RUOG SDQFUHDWLF FDQFHU GD\ • Evaluation of in vivo anti - tumor efficacy of LP - 184 in combination with ( i ) selected SOC chemotherapeutic agents (ii) radiation in xenograft models • Complete IND enabling studies in first half of 2022 • Plan on Phase 1 clinical trial in second half of 2022 Recent Highlights Upcoming Milestones 3XEOLFDWLRQ 3UHVHQWDWLRQ 37,700 (VWLPDWHG QHZ FDVHV LQ WKH 86 LQ Estimated Global incidence in 2021 Collaboration ”Synthetic lethality of LP - 184, a next generation acylfulvene, in ex vivo PDX models with homologous recombination defects” ”LP - 184, a novel alkylating agent, is highly effective in pancreatic cancers with DNA damage repair defects”
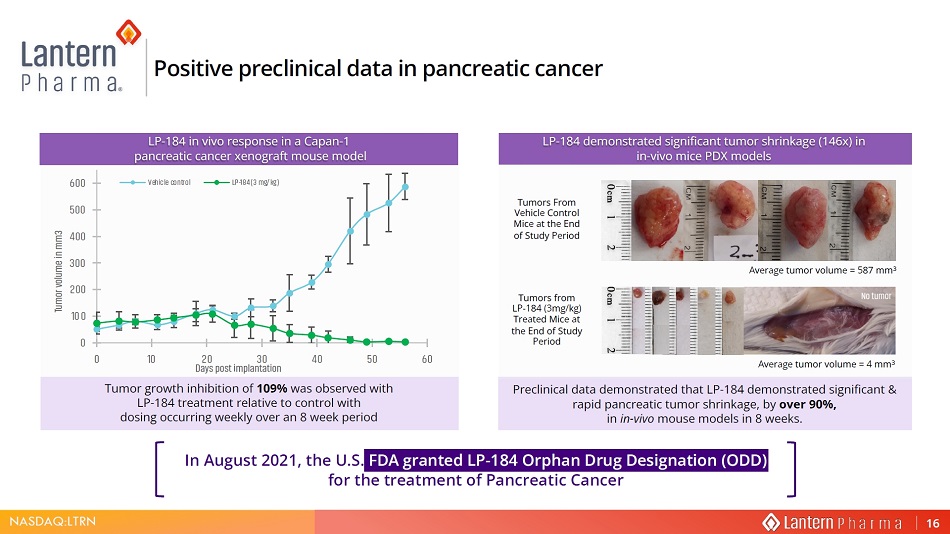
NASDAQ:LTRN 3UHFOLQLFDO GDWD GHPRQVWUDWHG WKDW /3 GHPRQVWUDWHG VLJQLILFDQW UDSLG SDQFUHDWLF WXPRU VKULQNDJH E\ RYHU LQ LQ YLYR PRXVH PRGHOV LQ ZHHNV Tumor growth inhibition of 109% was observed with LP - 184 treatment relative to control with dosing occur r ing weekly o ver an 8 week period In August 2021, the U.S. FDA granted LP - 184 Orphan Drug Designation (ODD) for the treatment of Pancreatic Cancer Positive preclinical data in pancreatic cancer 0 100 200 300 400 500 600 0 10 20 30 40 50 60 Tumor volume in mm3 ĂLJƐ ƉŽƐƚ ŝŵƉůĂŶƚĂƚŝŽŶ Vehicle control LP-184 (3 mg/kg) /3 LQ YLYR UHVSRQVH LQ D &DSDQ SDQFUHDWLF FDQFHU [HQRJUDIW PRXVH PRGHO LP - 184 demonstrated significant tumor shrinkage (146x) in in - vivo mice PDX models 7XPRUV )URP 9HKLFOH &RQWURO 0LFH DW WKH (QG RI 6WXG\ 3HULRG Average tumor volume = 587 mm 3 Tumors from LP - 184 (3mg/kg) Treated Mice at the End of Study Period Average tumor volume = 4 mm 3 No tumor
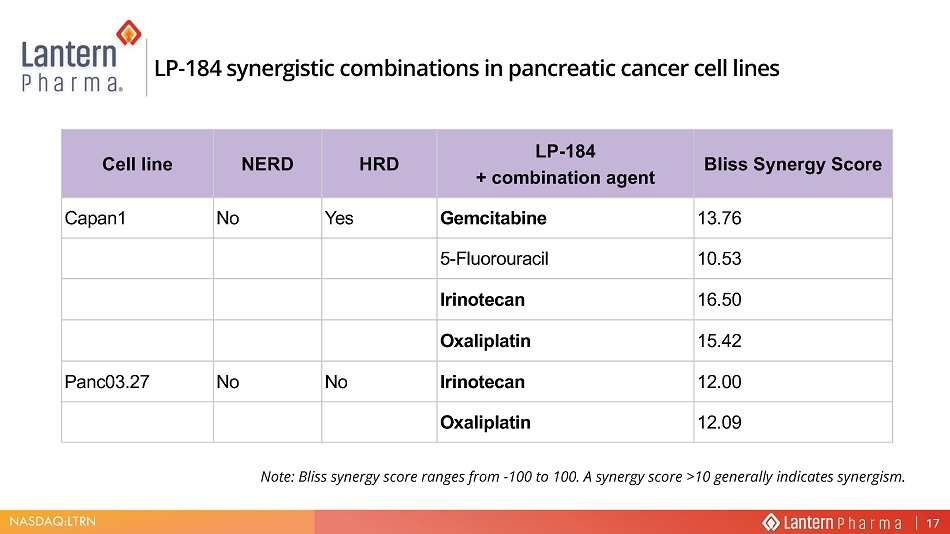
NASDAQ:LTRN &HOO OLQH NERD HRD LP - 184 + combination agent Bliss Synergy Score Capan1 No Yes Gemcitabine 13.76 5 - Fluorouracil 10.53 Irinotecan 16.50 Oxaliplatin 15.42 Panc03.27 No No Irinotecan 12.00 Oxaliplatin 12.09 Note: Bliss synergy score ranges from - 100 to 100. A synergy score >10 generally indicates synergism . /3 V\QHUJLVWLF FRPELQDWLRQV LQ SDQFUHDWLF FDQFHU FHOO OLQHV 17

NASDAQ:LTRN >W Ͳ ϭϴϰ Atypical Teratoid Rhabdoid Tumor (ATRT) • LP - 184 for ATRT granted Orphan Drug Designation and Rare Pediatric Disease Designation by FDA • ATRT is exceptionally sensitive to LP - 184, with response positively correlated to loss of SWI/SNF proteins that cause rhabdoid tumors and are altered in 20% of all cancers • Publications showing enhanced LP - 184 response with spironolactone combination and the effectiveness of LP - 184 in ATRT and other rhabdoid tumors are expected mid 2022 • Numerous Rhabdoid Tumors are believed to share ATRT sensitivity to LP - 184 and over 20 models are being tested at UT Health, with Dr. Peter Houghton • Protocol development for a Phase 1 trial in pediatric CNS cancers 5HFHQW +LJKOLJKWV Upcoming Milestones 60 (VWLPDWHG QHZ FDVHV LQ WKH 86 DQQXDOO\ Total existing cases of ATRT in the US 600 Collaboration 18
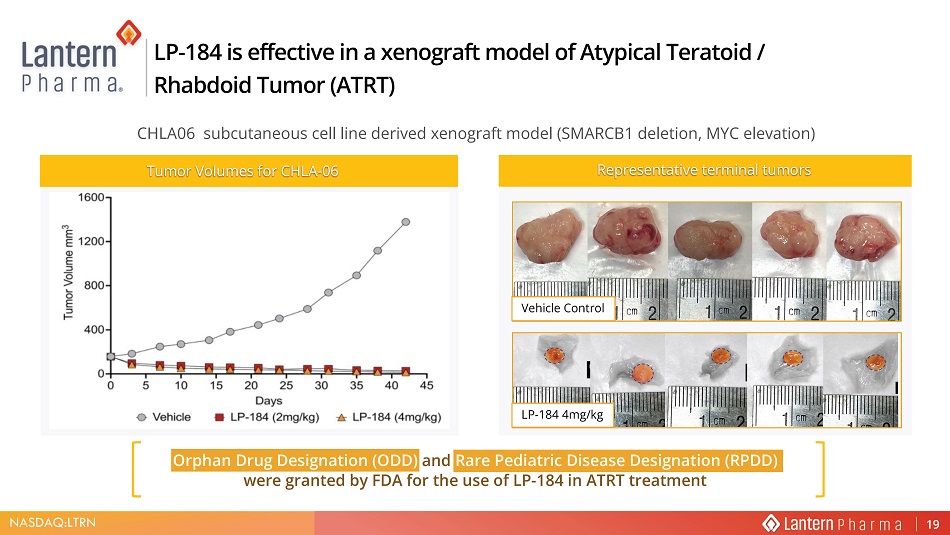
1$6'$4 /751 Orphan Drug Designation (ODD) and Rare Pediatric Disease Designation (RPDD) were granted by FDA for the use of LP - 184 in ATRT treatment LP - 184 is effective in a xenograft model of Atypical Teratoid / Rhabdoid Tumor (ATRT) 7XPRU 9ROXPHV IRU &+/$ Representative terminal tumors CHLA06 subcutaneous cell line derived xenograft model (SMARCB1 deletion, MYC elevation) Vehicle Control LP - 184 4mg/kg

NASDAQ:LTRN Highlights and Milestones ȏ 'HVLJQLQJ D OLEUDU\ RI $'& PROHFXOHV IRU IHDVLELOLW\ DQG SUHFOLQLFDO VWXGLHV R /LEUDU\ ZLOO EH GHYHORSHG ZLWK ERWK FOHDYDEOH DQG QRQ FOHDYDEOH OLQNHUV R 7KUHH SRWHQW SD\ORDGV EHLQJ FRQVLGHUHG ȏ ΖQLWLDO VWXGLHV ZLOO IRFXV RQ WKUHH SRWHQWLDO DQWLERGLHV WR WDUJHW HSLWKHOLDO DQG O\PSKRLG WXPRUV ȏ /HDG FDQGLGDWHV ZLOO EH FKRVHQ EDVHG XSRQ HIILFDF\ WR[LFLW\ E\VWDQGHU HIIHFWV DQG IOH[LELOLW\ RI SD\ORDGV WR DFKLHYH DQ DFFHSWDEOH UDQJH RI '$5 'UXJ $QWLERG\ 5DWLR Characteristics ADCs take advantage of the high potency of cytotoxic payloads and the superior specificity of antibodies. The drug antibody conjugate thus maximizes efficacy and minimizes systemic toxicity High Specificity 2 of the 4 largest oncology licensing deals in 2020 were for ADC assets AstraZeneca licensed a Ph 1 ADC from Daiichi Sanko for $6.0 billion Merck licensed a Ph2 ADC from Seagen for $3.2 billion WƌŽŐƌĂŵ Antibody Drug Conjugate (ADC) Program for Select Solid Tumors Growing
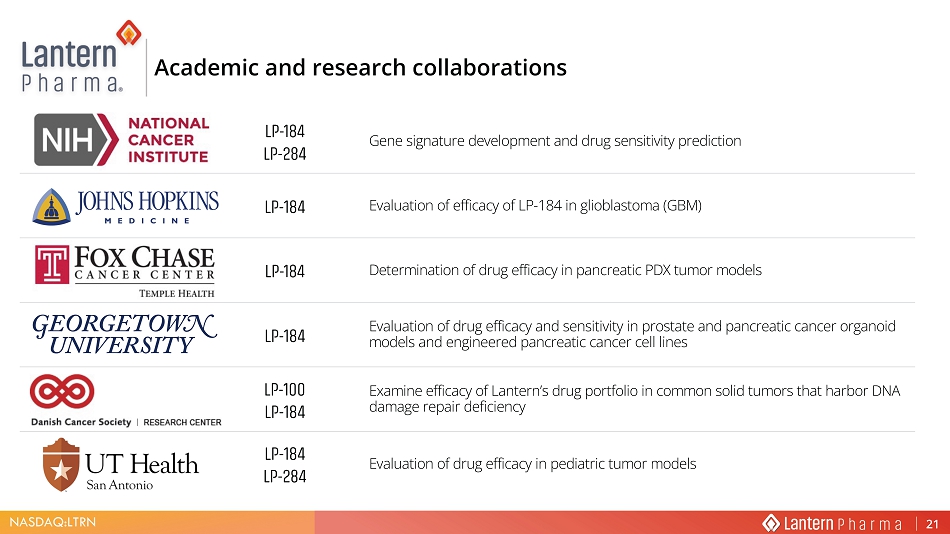
NASDAQ:LTRN LP - 184 LP - 284 Gene signature development and drug sensitivity prediction LP - 184 Evaluation of efficacy of LP - 184 in glioblastoma (GBM) LP - 184 Determination of drug efficacy in pancreatic PDX tumor models >W Ͳ ϭϴϰ Evaluation of drug efficacy and sensitivity in prostate and pancreatic cancer organoid models and engineered pancreatic cancer cell lines LP - 100 LP - 184 Examine efficacy of Lantern’s drug portfolio in common solid tumors that harbor DNA damage repair deficiency LP - 184 LP - 284 Evaluation of drug efficacy in pediatric tumor models $FDGHPLF DQG UHVHDUFK FROODERUDWLRQV 21

NASDAQ:LTRN 6WUDWHJLF FROODERUDWLRQ WR DFFHOHUDWH SDWLHQW HQUROOPHQW IRU WKH +DUPRQLF Œ & OLQLFDO 7 ULDO IRU QHYHU VPRNHUV ZLWK QRQ VPDOO FHOO OXQJ FDQFHU 16&/& XWLOL]LQJ /3 LQ FRPELQDWLRQ ZLWK FKHPRWKHUDS\ accelerate the patient enrollment predict outcomes and response in specific patient subsets Help Patients to have access to the right medicine at the right time 7HFKQRORJ\ FROODERUDWLRQV ZLWK 'HHS /HQV DQG &RGH 2FHDQ 22 Strategic collaboration to to facilitate the accelerated development of RADR ® while reducing development complexity and cost and increasing security and reproducibility /HYHUDJLQJ &RGH 2FHDQ ¶ V &RPSXWH &DSVXOH WHFKQRORJ\ • further power RADR ® platform for faster, more collaborative discoveries from billions of RADR data points, as well as data and insights from collaborators. • manage our external data and code collaborators with ease Further enhances our already established RADR ® platform and provides additional efficiencies in terms of development time and cost.

NASDAQ:LTRN We believe our solid financial position will fuel continued growth and evolution of our RADR ® A.I. platform, accelerate the development of our portfolio of targeted oncology drug candidates and allow us to introduce additional targeted product and collaboration opportunities in a capital efficient manner. ´ “ NASDAQ:LTRN

NASDAQ:LTRN Contents 01 Lantern Highlights Financial Overview 03 5 ' 8SGDWHV 04 Q&A 24
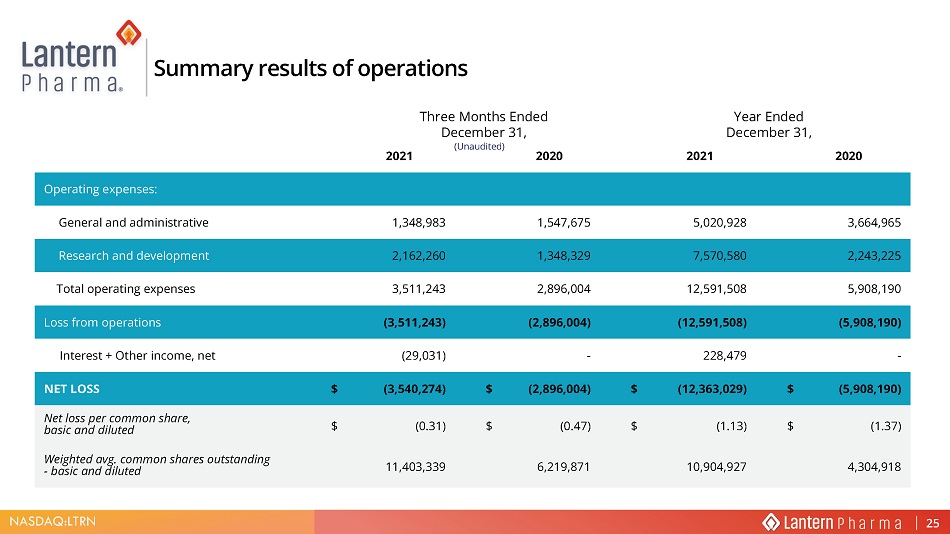
NASDAQ:LTRN Three Months Ended December 31 , Year Ended December 31, Summary results of operations 2021 2020 2021 2020 2SHUDWLQJ H[SHQVHV General and administrative 1,348,983 1,547,675 5,020,928 3,664,965 Research and development 2,162,260 1,348,329 7,570,580 2,243,225 Total operating expenses 3,511,243 2,896,004 12,591,508 5,908,190 Loss from operations (3,511,243) (2,896,004) (12,591,508) (5,908,190) Interest + Other income, net (29,031) - 228,479 - NET LOSS $ (3,540,274) $ (2,896,004) $ (12,363,029) $ (5,908,190) Net loss per common share, basic and diluted $ (0.31) $ (0.47) $ (1.13) $ (1.37) Weighted avg. common shares outstanding - basic and diluted 11,403,339 6,219,871 10,904,927 4,304,918 25 (Unaudited)
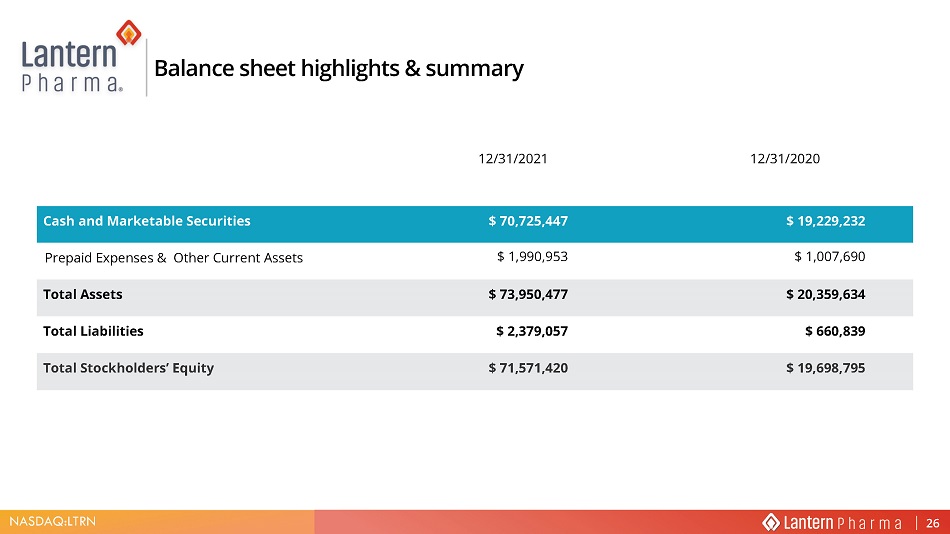
NASDAQ:LTRN Balance sheet highlights & summary 12/31/2021 12/31/2020 Cash and Marketable Securities $ 70,725,447 $ 19,229,232 Prepaid Expenses & Other Current Assets $ 1,990,953 $ 1,007,690 Total Assets $ 73,950,477 $ 20,359,634 Total Liabilities $ 2,379,057 $ 660,839 Total Stockholders’ Equity $ 71,571,420 $ 19,698,795 26
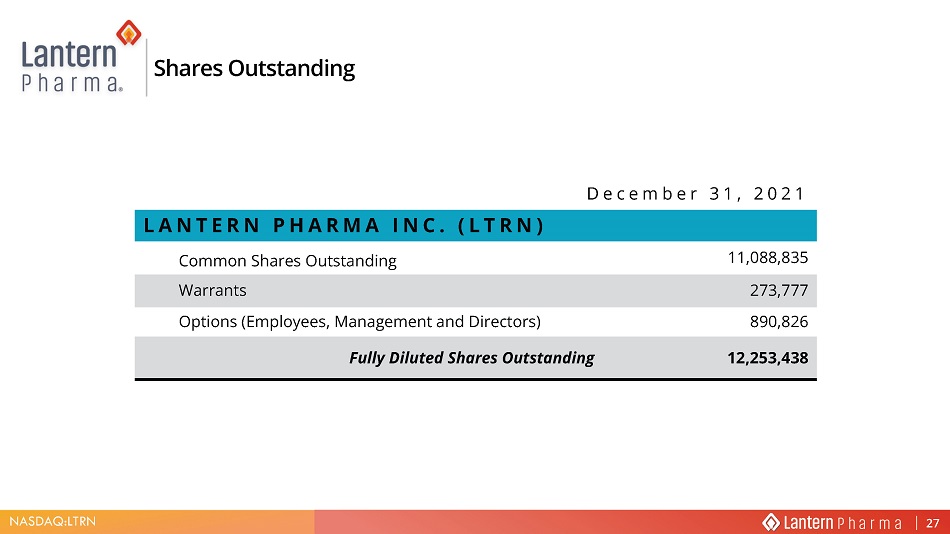
NASDAQ:LTRN Shares Outstanding December 31, 2021 LANTERN PHARMA INC. (LTRN) Common Shares Outstanding 11,088,835 Warrants 273,777 Options (Employees, Management and Directors) 890,826 Fully Diluted Shares Outstanding 12,253,438 27
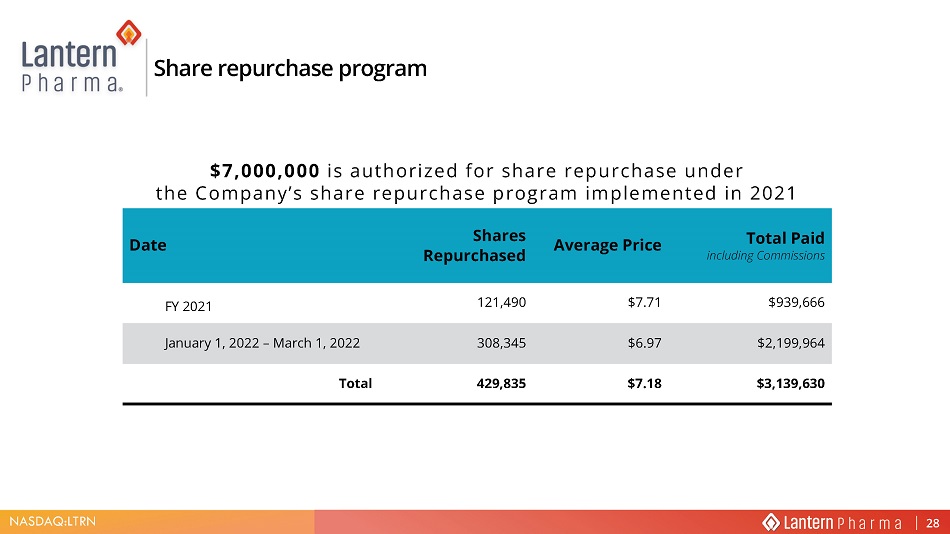
NASDAQ:LTRN Share repurchase program $7,000,000 is authorized for share repurchase under the Company’s share repurchase program implemented in 2021 Date Shares Repurchased Average Price Total Paid including Commissions FY 2021 121,490 $7.71 $939,666 January 1, 2022 – March 1, 2022 308,345 $6.97 $2,199,964 Total 429,835 $7.18 $3,139,630 28
NASDAQ:LTRN Migrating to a hybrid work environment HYBRID WORK ENVIRONMENT GROWING TEAM 29

NASDAQ:LTRN Contents 01 Lantern Highlights 02 Financial Overview 03 R&D Updates 04 Q&A 28 Contents 01 Lantern Highlights 02 Financial Overview 03 R&D Updates 04 Q&A 30
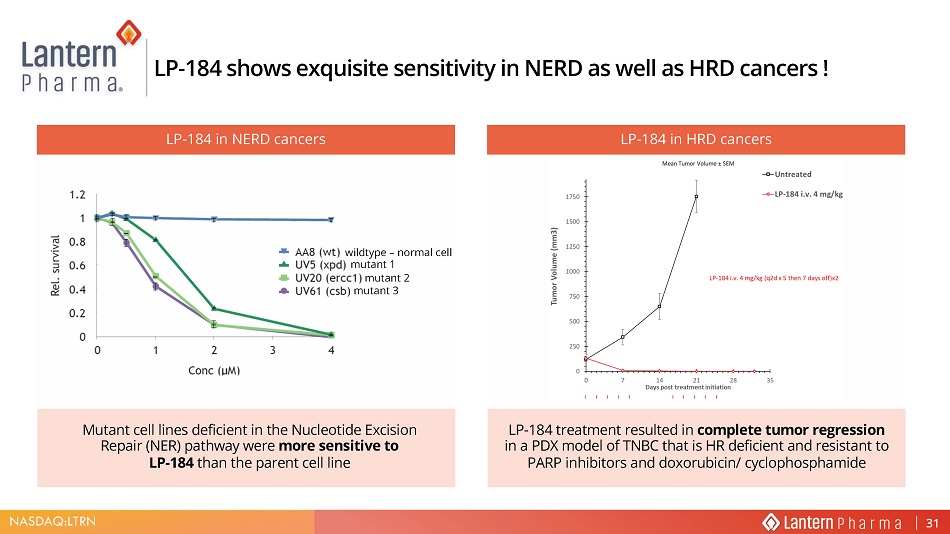
NASDAQ:LTRN wildtype – normal cell mutant 1 mutant 2 mutant 3 LP - 184 shows exquisite sensitivity in NERD as well as HRD cancers ! LP - 184 in HRD cancers LP - 184 treatment resulted in complete tumor regression in a PDX model of TNBC that is HR deficient and resistant to PARP inhibitors and doxorubicin/ cyclophosphamide LP - 184 in NERD cancers Mutant cell lines deficient in the Nucleotide Excision Repair (NER) pathway were more sensitive to LP - 184 than the parent cell line 31
NASDAQ:LTRN LP - 284 Mantle Cell Lymphoma • Showed nanomolar potency in a variety of hematological cancer cells including in mantle cell lymphoma, double - hit lymphoma, Burkitt's lymphoma, multiple myeloma, chronic myeloid leukemia, and acute lymphocytic leukemia • Presented at the 63rd ASH meeting and Exposition : chemical biology and experimental therapeutics • Investigate LP - 284’s potency in in vivo Mantle Cell Lymphoma models • Validate molecular biomarkers (unpublished data) that predict LP - 284 sensitivity • Examine LP - 284’s toxicity in animals Recent Highlights Upcoming Milestones Publication/ Presentation “The Positive Enantiomer of a Novel Chiral DNA Alkylating Agent Exhibits Nanomolar Potency in Hematologic Cancers” Jianli Zhou, Ph.D., Lantern Pharma 4,200 American Society of Hematology 2014 Estimated new cases in the US 2017 estimated Global incidence number 14,000 Collaboration 32
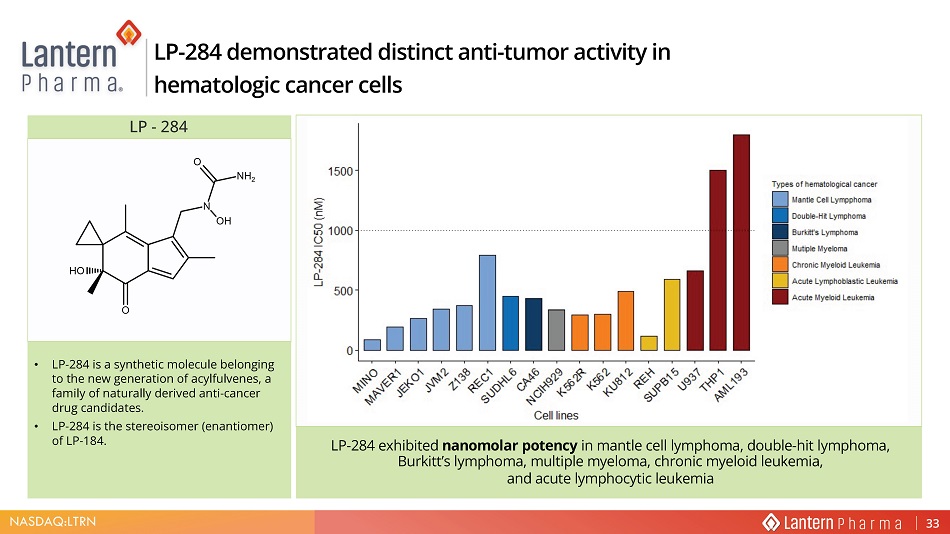
NASDAQ:LTRN LP - 284 demonstrated distinct anti - tumor activity in hematologic cancer cells LP - 284 • LP - 284 is a synthetic molecule belonging to the new generation of acylfulvenes, a family of naturally derived anti - cancer drug candidates. • LP - 284 is the stereoisomer (enantiomer) of LP - 184. LP - 284 exhibited nanomolar potency in mantle cell lymphoma, double - hit lymphoma, Burkitt’s lymphoma, multiple myeloma, chronic myeloid leukemia, and acute lymphocytic leukemia 33

NASDAQ:LTRN Ɣ /DXQFK RI 7KH +DUPRQLF Œ 7ULDO 3K FOLQLFDO WULDO IRU /3 LQ 16&/& Ɣ $GYDQFH /3 FOLQLFDO WULDO Ɣ /DXQFK 3K FOLQLFDO WULDO IRU /3 LQ JHQRPLFDOO\ GHILQHG VROLG WXPRUV Ɣ /DXQFK 3K FOLQLFDO WULDO IRU /3 LQ *%0 Ɣ 3URJUHVV /3 LQ $757 WRZDUGV 3K FOLQLFDO WULDO Ɣ $GYDQFH SHGLDWULF FDQFHU GUXJ GHYHORSPHQW SURJUDP Ɣ $GYDQFH $'& SUHFOLQLFDO VWXGLHV WR VXSSRUW IXWXUH 3KDVH ODXQFK Ɣ ([SORUH SRWHQWLDO FRPELQDWLRQV IRU /3 /3 /3 ZLWK RWKHU H[LVWLQJ DSSURYHG GUXJV Ɣ 6WUDWHJLFDOO\ JURZ 5$'5 p $ Ζ SODWIRUP WR ELOOLRQ GDWDSRLQWV Ɣ ([SORUH OLFHQVLQJ DQG SDUWQHUVKLS RSSRUWXQLWLHV A T ransformational year for Lantern 2022 34 Objectives

NASDAQ:LTRN Contents 01 /DQWHUQ +LJKOLJKWV 02 Financial Overview 04 Q&A 03 R&D Updates 35

1$6'$4 /751 Nasdaq: LTRN IR Contact: IR@lanternpharma.com 1 - 972 - 277 - 1136 www.lanternpharma.com @LanternPharma